Medicines and Healthcare products Regulatory Agency
Regulatory Agency
- Alerts, recalls and safety information: medicines and medical devices
- Drug Safety Update
- Yellow Card: Report a problem with a medicine or medical device
- Marketing authorisations and licensing guidance
- Product information about medicines
- Regulating medical devices
- Latest information for patients
- About MHRA
- All MHRA services and information
Featured
MHRA approves UK’s first new type of antibiotic for urinary tract infections in nearly 30 years
Press release
As with any medicine, the MHRA will keep the safety of gepotidacin under close review.

As with any medicine, the MHRA will keep the safety of zuranolone under close review.

The pilot will help sponsors prepare for a new substantial modifications process under upcoming regulations, with responses delivered within 14 days.

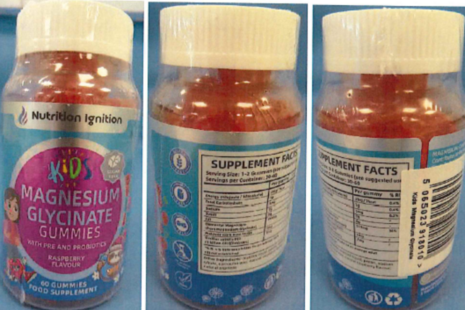
Parents and caregivers who have purchased Nutrition Ignition Kids Magnesium Glycinate Gummies should stop giving them to children and safely dispose of any remaining product. It is recommended that advice be sought from a healthcare professional if a child has any side effects that are of concern.
As with any medicine, the MHRA will keep the safety and effectiveness of teplizumab under close review.

Health Institution Exemption – Stakeholder survey
Open call for evidence
The MHRA invites health institutions in Great Britain to share their experience of the health institution exemption (sometimes referred to as an in-house manufacturing exemption).

Latest from the Medicines and Healthcare products Regulatory Agency
Subscriptions
What we do
The Medicines and Healthcare products Regulatory Agency regulates medicines, medical devices and blood components for transfusion in the UK.
MHRA is an executive agency, sponsored by the Department of Health and Social Care.
Follow us
Documents
Transparency and freedom of information releases
Our management








Contact MHRA
General enquiries
10 South Colonnade
London
E14 4PU
United Kingdom
Telephone
020 3080 6000
Fax
020 3118 9803
Office hours are Monday to Friday, 9am to 5pm.
Media enquiries
MHRA10 South Colonnade
London
E14 4PU
United Kingdom
Telephone (including out of hours):
020 3080 7651
Make an FOI request
- Read about the Freedom of Information (FOI) Act and how to make a request.
- Check our previous releases to see if we’ve already answered your question.
- Make a new request by contacting us using the details below.
Freedom of Information
10 South Colonnade
London
E14 4PU
United Kingdom
